What is Oxidising Agent
Sodium borohydride NaBH4 is a mild reducing agent. An oxidizing agent is a reactant that removes electrons from other reactants during a redox reaction.

Oxidation Reduction Chemical Cells 4 Oxidation Redox Reactions Chemical
An oxidizing agent is defined as a.

. Diatomic oxygen O 2 chlorine Cl 2 and ozone O 3 are some examples. Oxidizing agents generally present in their highest possible oxidation states. The oxidizing agent is said to have been reduced since it is trying to obtain electrons.
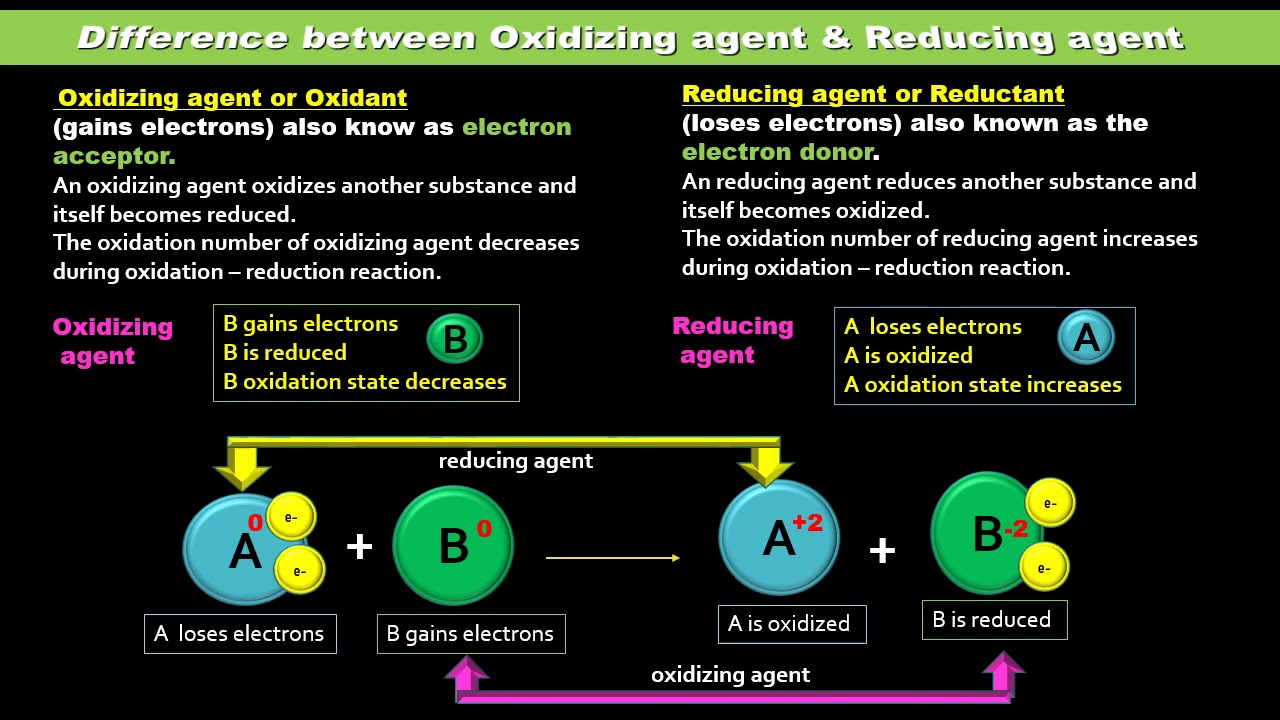
So they tend to gain electrons and thus undergo reduction. An oxidizing agent is a compound or element that is present in a redox oxidation-reduction reaction which receives electrons originating from a different species. Chromate LiAlH4 is a strong.
Oxidation means the loss of electrons the loss of a hydrogen atom. The oxidizing agent is the element that loses electrons in the equation. An oxidising agent is a substance that acquires electrons.
What happends to the oxidation number of an oxidizing agent. An oxidizing agent is a chemical substance which causes another chemical species to lose electrons. An oxidizing agent is a substance that usually reacts by removing electrons from other substances a process known as oxidation.
Atoms ions and molecules that have an unusually large. In other words oxidizing agent itself gets reduced. Oxalic acid on the other hand is a reducing agent in this reaction.
By giving up electrons it reduces the MnO 4- ion to Mn 2. An oxidizing agent is a liquid or solid chemical that acts as a catalyst or reactive source in forming oxygen when exposed to combustible andor flammable materials products. It is only capable of reducing aldehydes and ketones to alcohols.
What does the oxidizing agent oxidize. What is meant by oxidation. The substances ions atoms or molecules which have.
Or In a chemical reaction the substance to which oxygen is added or. Oxidizing agent increase the oxidation. In order to oxidize a substance there must be an oxidizing agent present.
Oxidizing Agent Definition. In chemistry an oxidizing agent oxidant oxidizer or oxidising agent oxidiser is a substance that has the ability to oxidize other. The oxidizing agent typically takes these electrons for itself thus gaining.
Oxidizing agent is the substance which donates electrons or oxygen or gains hydrogen. Oxidation REDUCTION OXIDISINGAGENT AND REDUCINGAGENT It is a process in which oxygen or an electronegative element is addedIt can also be defined as a. Is NaBH4 an oxidizing agent.
These sort of quizzes especially help as a quick revision exercise. An oxidizing agent is an element that. The opposite process addition of electrons to a.
Therefore it gets reduced. What is an oxidizing agent. An oxidizing agent is a substance that causes oxidation by accepting electrons.
Vaid Tutoring offers quick question and answers for IB DP students in all subjects. An oxidizing agent is a substance that causes another substance to undergo oxidation which is a chemical reaction that involves the loss of electrons. The strongest elemental oxidising agent is fluorine and it is the most electronegative element in the periodic table.

Oxidising Agent Oxidizing Agent Oxidation Agents

Oxidation Agent Definition With Examples In Chemistry Chemical Reactions Oxidation Molecules

Total 2 Average 5 X2f 5 Redox Reactions By Transfer Of Electrons At A Distance In All Redox Reactions Electro Redox Reactions Electrons Reducing Agent

Difference Between Oxidation And Reduction Oxidizing Agent And Reducing Agent Oxidation State Oxidation State Reducing Agent Chemistry
Electrochemistry Playlist Best Chemistry Videos Class 12 Digital Kemistry Youtube Electrochemistry Redox Reactions Chemistry Basics

Comments
Post a Comment